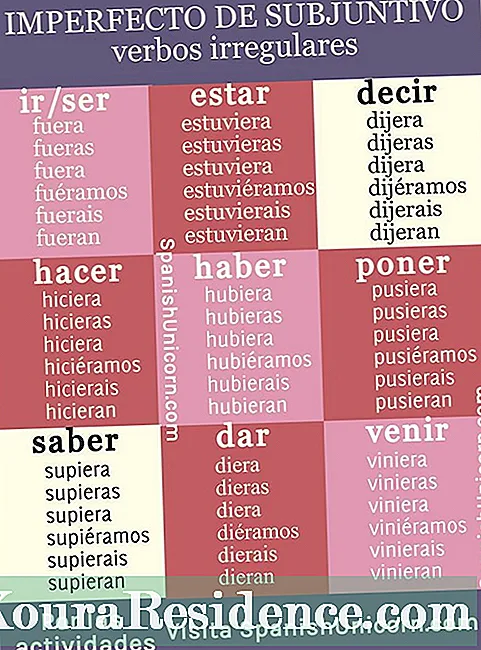
The chemistry It is the science that studies the composition and the transformations that can occur to matter, in any of its forms. One of the most important areas of study in chemistry is that of gases, as it is necessary to carry out an analysis of their behavior on Earth.
Gases, as is intended throughout the discipline, should be explained by means of equations and other mathematical and statistical elements, which in any case are different depending on the type of gas and the conditions around it. Due to the complexity of these calculations, the chemist Jan van Helmont (the same one who coined the concept of gas) drew up a famous Law, which generalizes a tendency to gas behavior, in its relationship between kinetic energy and temperature.
The Van Helmont's Law, in its simplest version, indicates that at constant temperature the volume of a fixed mass of gas is inversely proportional to the pressure that it exerts: P * V = k constant. However, like any scientific contribution, it must be able to be collated and its reliability guaranteed, which was found not to occur in all cases.
The conclusion reached is that it is not that the Law was wrong, but that it only worked for a theoretical gas, an assumption of gas in which the molecules do not collapse between them, always has the same number of molecules that occupy the same volume at the same conditions of pressure and temperature, and has no attractive or repulsive forces.
The ideal gas, despite not representing a gas that really exists, it is a tool to facilitate a large number of mathematical calculations.
The general equation of ideal gasesFurthermore, it results from the combination of two other fundamental laws for chemistry, which also assumes that gases comply with the characteristics of ideal gases. Boyle-Mariotte's Law relates the volume and pressure of a quantity of gas at constant temperature, seeing that they are inversely proportional. The Law of Charles - Gay Lussac relates the volume and the temperature, seeing that they are directly proportional with constant pressure.
It is not possible to create a concrete list of ideal gases, because as said it is a unique hypothetical gas. If you can list a set of gases (including noble gases) whose treatment can be identical to that of ideal gases, because the characteristics are similar, as long as the pressure and temperature conditions are normal.
- Nitrogen
- Oxygen
- Hydrogen
- Carbon dioxide
- Helium
- Neon
- Argon
- Krypton
- Xenon
- Radon
The real gases they are, in opposition to the ideals, those that have a thermodynamic behavior and therefore do not follow the same equation of state as the ideal gases. In high pressure and low temperature, gases must inevitably be regarded as real. In that case the gas is said to be at a high density condition.
The substantial difference between ideal gas and real gas is that the latter cannot be compressed indefinitely, but its compression capacity is relative to the pressure and temperature levels.
The real gases they also have an equation of state that describes their behavior, which is the one provided by Van der waals in 1873. The equation has a fairly high feasibility under low pressure conditions, and modifies the ideal gas equation to some extent: P * V = n * R * T, where n is the number of moles of the gas, and R a constant called 'gas constant'.
Gases that do not behave in a way similar to ideal gases are called real gases. The following list shows some examples of these gases, although those that have already been listed as ideal gases can also be added, but this time in a context of high pressure and / or low temperature.
- Ammonia
- Methane
- Ethane
- Ethene
- Propane
- Butane
- Pentane
- Benzene