Author:
Laura McKinney
Date Of Creation:
2 August 2021
Update Date:
1 July 2024

Content
It is called catalysis to the chemical process of acceleration or slowing down of a chemical reaction, from the addition of a substance or element, both simple and compound, that alters reaction times without affecting the nature of the final product of the same and, in addition, without losing its own mass in the process, which it does occurs with reagents.
This element is called catalyst. Each chemical reaction has a suitable catalyst, which can accelerate, magnify or enhance (positive catalyst), or on the contrary slow down, decrease and weaken (negative catalyst) your process. The latter are often known as inhibitors.
See also: Examples of Catalysts (and their functions)
Examples of positive catalyst
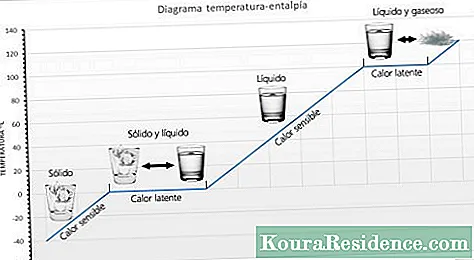
- Temperature. Most chemical reactions can be accelerated without altering their products, just by increasing the temperature of the reaction medium. For this reason the decomposition of the matter occurs most rapidly in the tropics.
- Enzymes. Naturally segregated by the body of living beings, enzymes play an important catalytic role, accelerating vital processes that, if they occurred on their own, would require temperatures often incompatible with life. (see: digestive enzymes)
- Palladium catalysts. For automobiles that use unleaded gasoline, pipes with palladium or platinum in small particles adhere to the exhaust of the automobiles, can catalyze the process of attenuation of carbon monoxide and other toxic gases of combustion, allowing to reduce them to substances less dangerous in record time.
- Fluorine derivatives. They accelerate the decomposition of ozone (O3 → O + O2) in oxygen, a reaction that is normally slow. This is the problem of aerosols and refrigerants that release CFCs into the atmosphere: they catalyze the ozone layer in this sense.
- Magnesium dioxide (MnO2). A frequent catalyst in the decomposition of Hydrogen Peroxide or hydrogen peroxide (2H2OR2 → 2H2O + O2) in water and oxygen.
- Nickel. Used in the hydrogenation of vegetable oils, to obtain margarine, as this metal accelerates the process of obtaining saturated lipids.
- Silver. Polycrystalline silver and nanoporose are effective accelerators of carbon dioxide (CO2) by electrocatalysis.
- Aluminum chloride. Employee at industry petrochemical industry to speed up the production of synthetic resins or lubricants, without altering the delicate nature of hydrocarbons in question, since it has acidic and basic properties at the same time (amphoteric substance).
- The iron. It is used as a catalyst in the Haber-Bosch process to obtain ammonia from hydrogen and nitrogen.
- UV light. UV light, along with a specific catalyst, composes photocatalysis: the acceleration of a chemical reaction by the work of a catalyst activated by the light energy of ultraviolet.
Examples of negative catalyst
- Temperature. Just as the increase in temperature accelerates the chemical processes, its decrease delays them. This is the principle of refrigeration, for example, which prolongs the life of food by keeping it at a low temperature.
- Citric acid. The acid of lemon and other citrus fruits slows down the oxidation process of the organic material.
- Enzyme inhibitors. Biological substances that bind to enzymes and reduce their activity, to stop chemical or biological processes. They are often used to combat pathogenic microorganisms, inhibiting some key process for its reproduction.
- Potassium chlorate. Used in blueing processes, in which magnetite steel is coated to slow down or prevent its corrosion process.
- Sorbic acid. Natural preservative used in the food industry to slow the decomposition of food.
- Tetraethyl lead. In the now extinct leaded gasoline, this substance was used as an antiknock, that is, to prevent its premature explosion.
- Propanoic acid. A colorless, corrosive liquid with a pungent odor, it is conducive to preserving feed, food and pharmaceutical products, as it is a powerful antifungal and mold growth inhibitor.
- Sulfur and derivatives. These compounds act as inhibitors of the positive catalysis of powdered platinum or nickel in hydrogenation reactions. The appearance of sulfur stops the effect and returns to its normal reaction rate.
- Hydrocyanic (or prussic) acid. Highly toxic, its effect on animals or humans interrupts the process of numerous metalloenzymes, thus preventing cellular respiration and causing death in a few minutes.
- Mercury, phosphorus, or arsenic vapor. These substances completely cancel out the action of platinum asbestos in the manufacture of sulfuric acid, acting as a powerful inhibitor.